Bioink for 3D-Bioprinting with Decellularized Bone Particles:
Biofabrication is a technology that uses extrusion printing methods to create three-dimensional (3D) living tissue structures. One of the key challenges in biofabrication is maintaining the shape fidelity of the 3D-printed constructs while allowing cell proliferation. Material characteristics directly affect printability, shape fidelity, and cell behavior post-bioprinting. Gelatin (GEL), due to its cytocompatibility, enzymatic degradability, and other beneficial properties, is often used in this context.
The incorporation of decellularized bone (DB) particles into the bioink formulation can significantly enhance the outcome of biofabrication. DB particles, derived from natural bone extracellular matrix (ECM) proteins and the biomineralization property of bone tissue, can enhance the proliferation and osteogenic differentiation of cells within the bioink formulations when used as an additive in the GEL matrix. This is because the natural bone ECM proteins and the biomineralization property of bone tissue can provide a more natural environment for the cells, thereby enhancing their behavior.
A recent study done by researchers from the Izmir Institute of Technology, Friedrich-Alexander-University Erlangen-Nuremberg and Technische Universität Dresden, aimed to develop a minimalistic bioink formulation composed of GEL, DB particles, and cells, and evaluate the effect of different DB particle sizes and concentrations within the GEL matrix on cell behavior. The study tested the GEL/DB composite bioink formulations with mouse pre-osteoblasts and human telomerase reverse transcriptase-immortalized mesenchymal stem cells. The hypothesis was that reducing the particle size may increase printability and cell behavior could improve with higher DB particle concentration.
The GeSiM’s BioScaffolder 3.1 was used in the fabrication of 3D cylindrical-shaped cell-laden hydrogels. The bioinks were transferred into the cartridge of the bioprinter and extruded through the nozzles to create the desired 3D structures. Then, the effect of different sizes of DB particles in two different bioink formulations with different cell types was evaluated. All 3D bioprinted cell-laden GEL/DB constructs were evaluated in terms of cytocompatibility, bioactivity, and osteogenic differentiation throughout a 28-day culture period.
Finally, this study demonstrated that a GEL bioink formulation with smaller DB particles (45 μm) allowed for better 3D printing with a higher particle concentration (10%) and increased the viscosity and shape fidelity of the bioink, making it a promising formulation for 3D bioprinting applications in bone tissue engineering. Future work could focus on in vivo studies and gene expression analysis to further assess the functionality of these bioinks.
We’re proud of GeSiM’s Bioscaffolder 3.1 role in this research as it provided the platform for the 3D bioprinting of the bioinks into cell-laden hydrogels. This highlights the importance of precise and reliable bioprinting technology in the field of biofabrication.
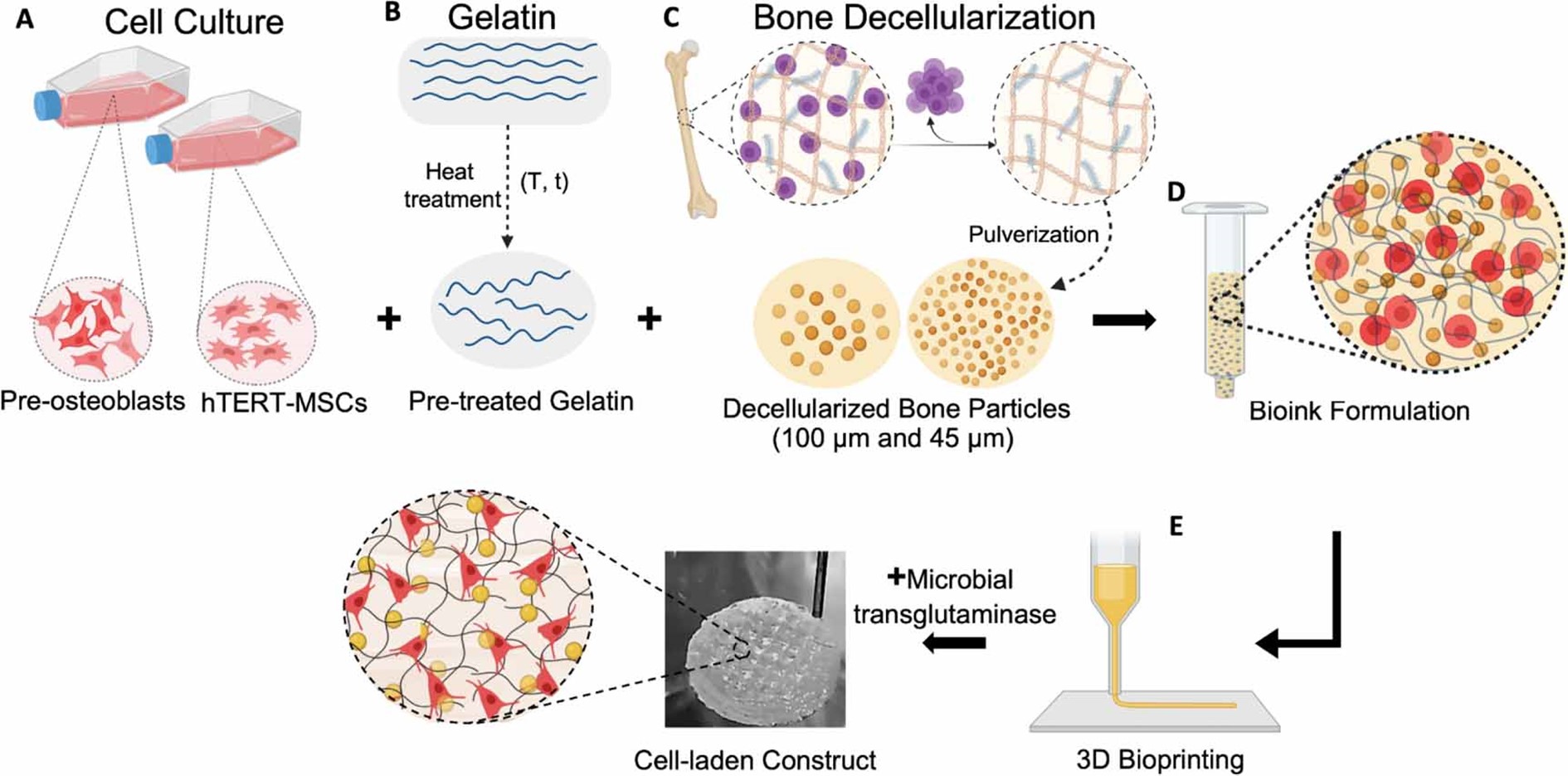
Figure 1. Schematic illustration of the study. (A) MC3T3-E1 pre-osteoblasts and hTERT-MSCs were used for bioink preparation. (B) GEL was prepared, and heat treated to enhance its rheological properties. (C) To prepare tissue-based additives, animal derived bone tissue was decellularized and particles were obtained in different sizes (100 µm and 45 µm) by pulverization. (D) For bioink formulation, cells, pre-treated GEL and DB particles were mixed and (E) cell-laden 3D constructs were fabricated by the 3D bioprinting technique. (Created with BioRender)
Aylin Kara Özenler et al. 3D bioprinting of mouse pre-osteoblasts and human MSCs using bioinks consisting of gelatin and decellularized bone particles. Biofabrication. 2024,16. 025027. https://doi.org/10.1088/1758-5090/ad2c98
