MicCell – Microfluidics on the Microscope Stage
MicCell – Customizable Tool Kit for Microbiology
Microfluidic systems may integrate fluidic, electronic and optical functions. Being a valuable tool in routine use prototyping can be challenging and expensive. When diagnostic cartridges needs to be optical transparent they often feature hot embossed microplastics. The manufacturing processes than require expensive production machines and layout specific casting tools.

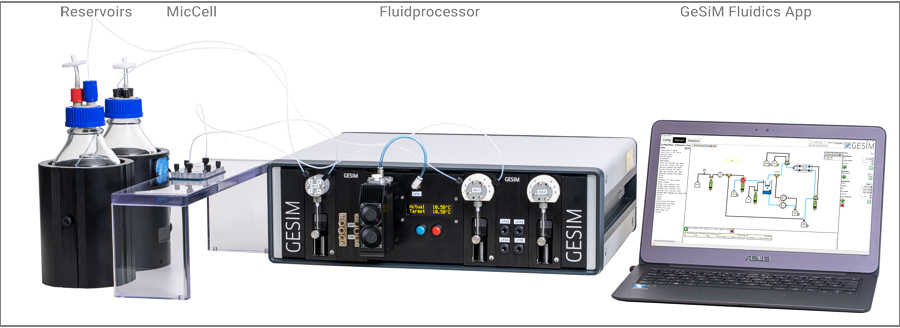
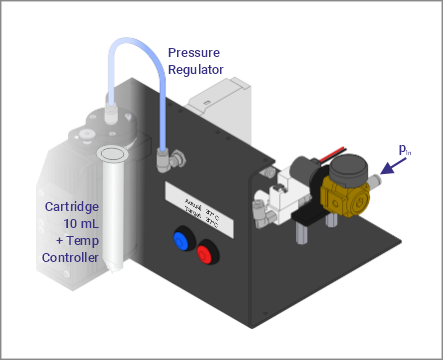
The GeSiM MicCell on a microscope stage (left), attached to a FluidProcessor (mid), controlled by GeSiM Fluidics app (right)
For R/D purposes the MicCell Microscopy Cell combines an affordable casting process using PDMS and a convenient mechanical setup for quick replacement of the used PDMS channel plate. The mechanical frame of the MicCell fits on standard microscope stages.
Please also refer to the Microfluidics section for silicon-glass flowthrough cells and complex microfluidic instruments.
PDMS Flow-Cells: The “Do-It-Yourself” Way
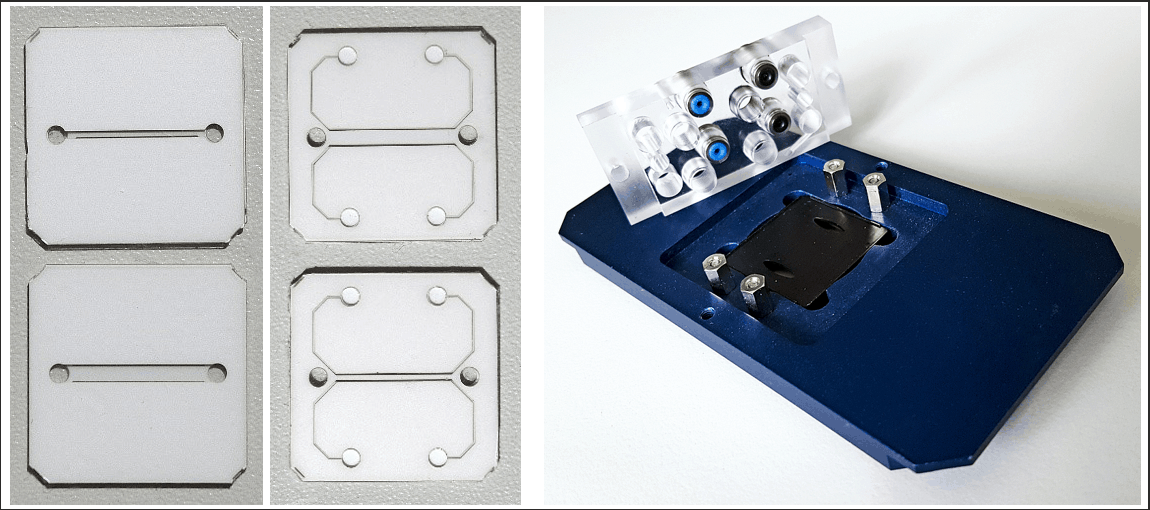
Core of the MicCell is an optical transparent PDMS (Polydimethylsiloxane) chip with a user specific microfluidic channel layout. It is accomplished by “channel spacers” for fluidic interconnections and calottes/ polycarbonate frames for mechanical adaptation to standard microscope systems.
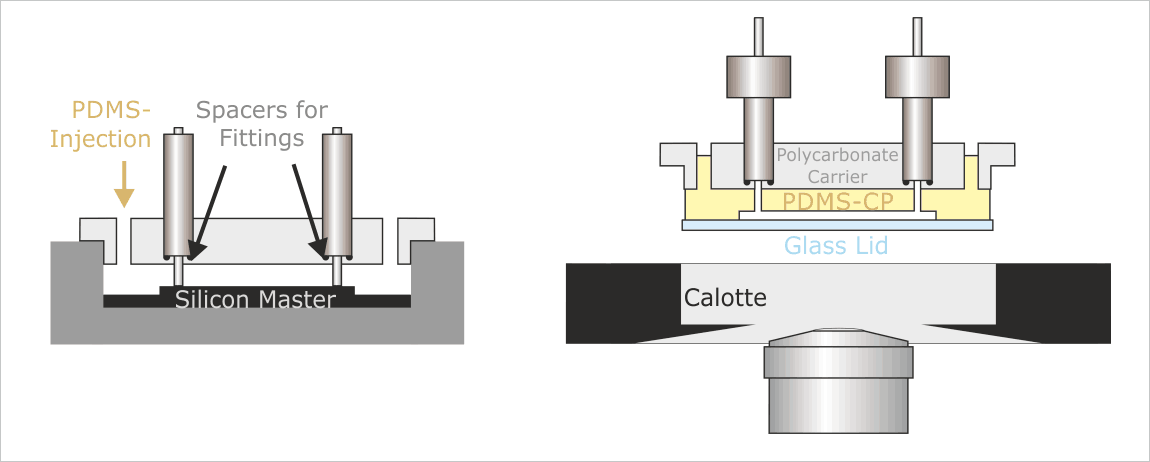
The “MicCell-Process”: Preparation of the PDMS-CP “Channel Plate” (Left); Position of the PDMS-CP during usage (Right)
Standard sizes for the PDMS channel plate are:
- 22 x 22 mm
- 22 x 50 mm
- 25 x 75 mm
The PDMS channel plate goes in between the Polycarbonate carrier and the calotte and gets sealed by a standard glass lid. Glass lids wih electrode structures on the inner side are available on request.
On top of all, the MicCell channel plate carrier can be completed with other microfluidic parts, tubes, filters, bottles, syringe systems and an operation software. Move on to FOILS VS. PDMS for more information and downlaod the full MicCell catalogue on the bottom of this page.
Casting Set for PDMS Channel Plates
The casting set mainly comprises the casting station, Polycarbonate carriers, liquid PDMS and syringes. In addition, you will need a “Master Chip” introducing your particular channel layout.

Casting set for PDMS channel plates (CP)
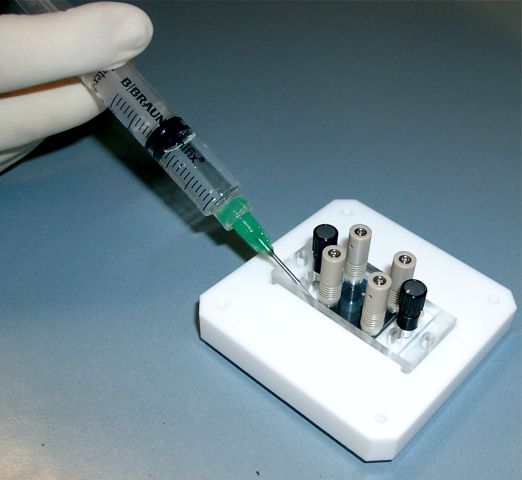
Injection of the liquid PDMS into the casting station
The master chip is made from Teflon coated silicon and made at GeSiM on request. Basically we accept any layout provided by a CAD drawing; a set of typical channel layouts is on the shelf. Please ask.
Foil Based Flow-Cells: Made to Order
Alternatively GeSiM offers customized microfluidic systems made of adhesive foils that are cut by different technologies and assembled in our factory. They can be ordered in small and large quantities, thus can be used as disposable, and snugly fit in the GeSiM MicCell chamber. They are an attractive alternative to PDMS channel plates. GeSiM is currently redesigning the BSyS robot to produce them automatically.
Systems from glass are also available, please inquire.
Intricate multi-layer layouts can be achieved so that numerous applications, e.g. point-of-care diagnostics, become feasible. Interesting accessories are membranes with defined cylindrical nanopore geometries from Oxyphen AG, produced by beaming with energetic heavy ions. This has e.g. allowed the safe encapsulation of genetically engineered yeast cells in biosensors for anthropogenic pollutants in wastewater (Schirmer et al., 2018, 2019; Günther et al., 2019).

“Track-etched” membrane (Oxyphen GmbH) placed on a cut foil (darker) with round hole and channel. The speckles are the pores.
Measurement of Tiny Flows
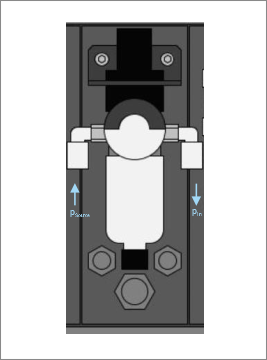
The GeSiM flow sensor has been designed for flow rates in the range of 0…100 Microliters per minute. It is a thermocalorimetric sensor measuring the heat transfer in a very tiny chamber. The fluid sample is negligibly heated whereas the surroundig temperature distribution is monitored.
As larger the heat transport through the chamber as more precisely the measurement result will be. In other terms, as more time is allowed for a single measurement as better the accuracy.
The sensor can be used along with the MicCell Fluid Processor (See tab FLUIDPROCESSOR) but is available for standalone operation too. The sensor is cascadable, it allows to measure several independent fluid flows simultaneously. Up to four stacked sensors can be managed by a single controller. The controller connects to a PC through serial interface, a dedicated software displays the measurement result.
Introduction
Fluid automation at the benchtop format can expedite your daily work in the lab. Whether you operate at the micro- or macroscale, the GeSiM FluidProcessor (FP) is your personal central processing unit for liquids and gasses. The FP is a horizontal rack with standardized slots for functional modules. Each FluidProcessor is custom made to order, configured for your particular application. The FP builds up from a library of functional modules, thus the users will get exactly the hardware for their special purpose. The ability of post-sales reconfiguration adapts your FluidProcessor to changing tasks, securing sustainability and investment protection.
The FluidProcessor hooks up to your microfluidic system. The optional combination with a GeSiM “MicCell” flow-cell (see tab FLOW-CELL) results in a fully automated microfluidic setup for many applications in life science and chemistry. Standard fittings (Upchurch Scientific and others) allow an easy connection to other flow-cells, e.g. from Microfluidic Chipshop.
The FluidProcessor and GeSiM Robots
The GeSiM FluidProcessor and the GeSiM robots share the same automation platform.
Whilst the FluidProcessor operates microfluidic cartridges with openings, semipermeable lids, open wells on deck of a GeSiM instrument, it can interact with tool heads for pipetting, 3D printing, or camera inspection installed on the robot. 3D Printing of organoids in chambers of fluidic cartridges, sealing, cell cultivation and camera inspection can be done at one place without moving sensitive targets through non-sterile environments.
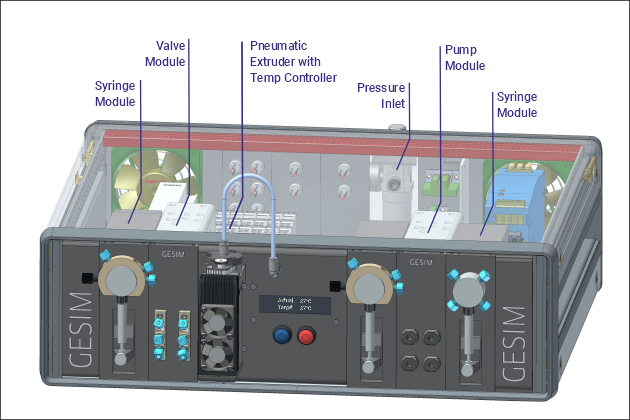
Library of Functional Modules for the GeSiM FluidProcessor
How to get hold of your individual GeSiM FluidProcessor?
- Contact GeSiM with your application, drawings, charts, descriptions,
- Select functional modules of interest,
- GeSiM designs your FluidProcessor, completed with internal/ external tubes, filters and reservoirs. Fittings are available for GeSiM flow-cells as well as for your own microfluidic components. A detailed tubing scheme is available for the customer before your FP leaves our manufacturing facility.
What is available for the GeSiM FluidProcessor?
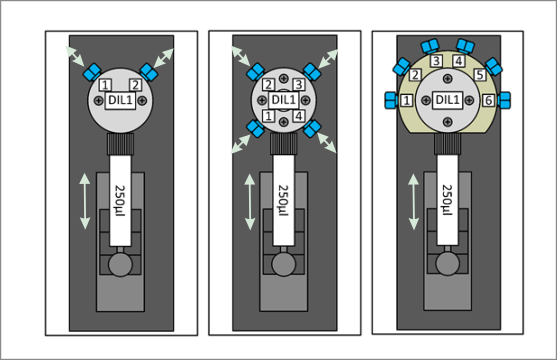
- Syringe pumps, with and without selector valves for precise displacement dispensing
- Membrane pumps for continuous liquid flow,
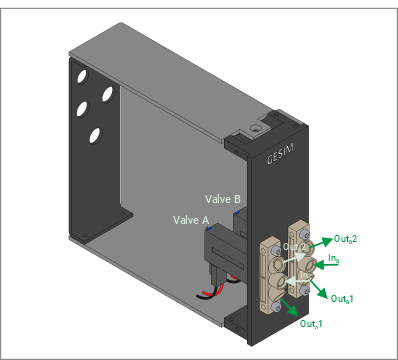
- Two- and three-way valves
- Pressure regulators for different pressure ranges
- Vacuum generator
- Liquid-level sensors for external reservoirs
- External reservoir bottles of different volumes (250 mL to 1,000 mL)
- Pneumatic extruder unit with cooler/ heater for 10ml cartridges,
- Drivers for piezoelectric drop-on-demand dispensers
- Drivers for chip-based micro-flow sensors (Measurement range below 70 µL/min)
Please ask for the MicCell catalogue with detailed information on all available functional modules.
Explore the Inside of a FluidProcessor
The FluidProcessor combines fluid handling modules in an application specific manner. The available modules at standardized width plug into the slots of the instrument. Modules will occupy either a single slot or multiple slots. Modules slide in on front or rear panel, depending on their function. Each FP comes with a CPU, power adapter, regulated fans and Ethernet based backplane. It hooks up to the control computer by a usual LAN cable.
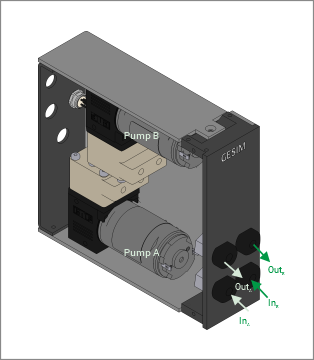
Double membrane pumps: The cost-effective solution for continuous liquid flow through flow cells. Max. flow about 0.3 L/min, max. pressure about 1 bar. Pumb chambers consists of FFKM/ PTFE. Other materials are available on request. Fix pump rate, the module comes with tube connectors ¼“-28 UNF (female).
Software GeSiM Fluidics
GeSiM Fluidics is the operation software of the GeSiM Fluidprocessor. Same as for the hardware, GeSiM Fluidics is modular. Once configured, it reflects exactly the hardware on your bench. A graphical user interface offers click-and-drop for functional modules, tubes, external reservoir bottles and (microfluidic) flow-cells.
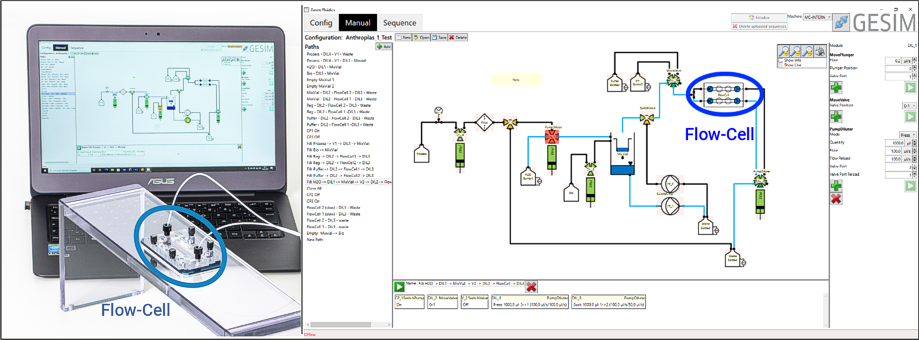
Combining micro- and macrofluidics: The software GeSiM Fluidics embeds your flow-cell into the functional environment of the FluidProcessor. A GeSiM MicCell (left, fixed on a PMMA rack), and its virtual counterpart on the graphical user interface of GeSiM Fluidics
GeSiM Fluidics is structured like a simple website and offers three main operational modes. Just move from left to right when working with your FluidProcessor.
Configuration: The available hardware modules and tube connections can be arranged to meet your particular application.
Manual Mode: Now the software requires the link to the FluidProcessor. After initialization, hardware components can be operated by mouse click. Simple “operational paths”, e.g., for pumping liquid into a waste reservoir, must be defined.
Sequence page: Create, save, and execute complex sequences for automatic runs and unattended operation.
The Versatile MicCell System: Research Already Done
The MicCell System gained much attention from researchers world wide. Here we present a list of possible and already done applications. Please contact us for further details.
- Micro-reaction technology, e.g., hybridization or stoppedflow chamber, using fluorescence detection
- Immobilization of biomolecules (e.g., protein or DNA) in the microchannels before assembly, e.g., by microarraying
- Generation of concentration gradients perpendicular to the microchannel cross section by a “gradient mixer”
- Semi-automatic drug screening using adherent cells or tissue slices
- Viability tests and other cell-based physiological assays
- Measurement of the interaction of cells with immobilized proteins, DNA, RNA, oligo- or polysaccharides, lipids, and other ligands
- Cell handling and sorting using optical tweezers
- Identification of cancer or stem cells using an “optical stretcher” (patent University of Leipzig)
- Electroporation in the flow
- Testing of the uniformity of microbeads and other particles, potentially with sorting
- Manipulation of elongated macromolecules (e.g., DNA or motor proteins) in hydrodynamic flow fields for the bottom-up construction of nanostructures, force measurements, etc.
- Micro-capillary electrophoresis under the microscope
- Integration of, e.g., column or filtration material for micro-purifying with or without microscope control
- Liquid processing independent of a microscope (e.g., assays using electrochemical detection)
Chemical synthesis on the nanoscale - Observation of opaque objects in the MicCell using the pivotable sample carrier
Below we present a few examples.
Wastewater Analysis Using Foil-Based Microfluidics
Together with Fraunhofer IKTS (Dresden) and other companies (BMBF project ANTHROPLAS), a nanoplasmonic biosensor for 24/7 detection of 10 µg/l diclofenac in wastewater was developed (Steinke et al., 2018). It features a functionalized nanostructured gold surface in a foil-based microfluidic system (Steinke et al., 2019) and a miniaturized optoelectronic unit (Wuchrer et al., 2016).

PC foil with four nanopillar arrays made by NIL
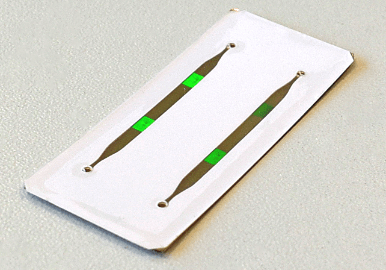
Ready-to-use chip with two 2 mm wide / 90 µm deep channels in a white laser-cut foil
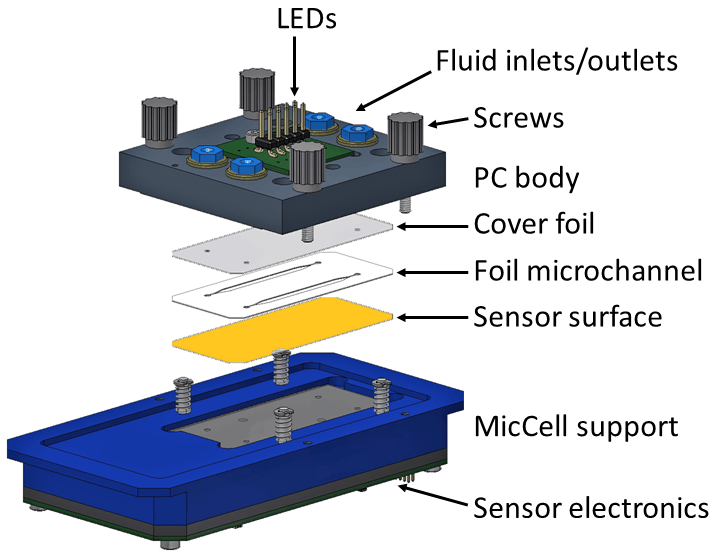
Exploded view of complete sensor system
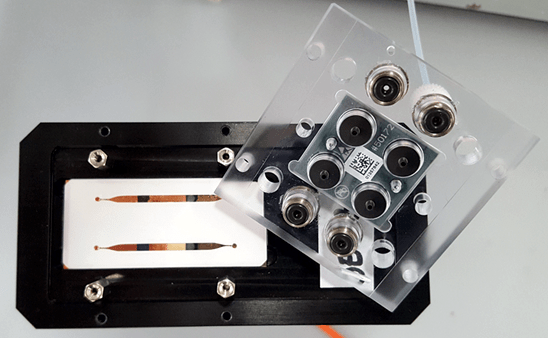
Sensor cell without PC body
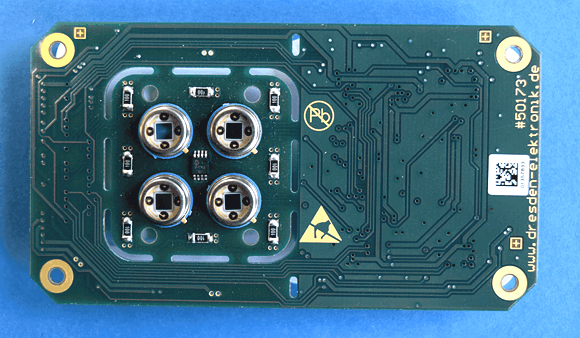
Four channel sensor electronics, placed under the fluidic channels (dresden elektronik)
GeSiM’s contribution:
- Nanopillar arrays in a UV-curable photoresist on a polymer substrate made by nanoimprint lithography with a GeSiM Microcontact Printer.
- Foil-based microfluidic systems, based on adhesive tape technology of Fraunhofer IKTS, with two flow channels operating in parallel.
- A casing for the microchannels with standard “chip-to-world” interface based on the GeSiM MicCell that also integrated the optoelectronic system.
- Software for easy control of all fluidic components.
Foil Based Multi-Layer Flow Cell to Study Gradients
Flow cells were designed in which a collagen layer with live cells is exposed to two different concentrations of an analyte to study the effect of chemical gradients on the fate, e.g. differentiation, of cells or organoids grown in extracellular matrix. A track-etched above the collagen prevents outflow of cells.
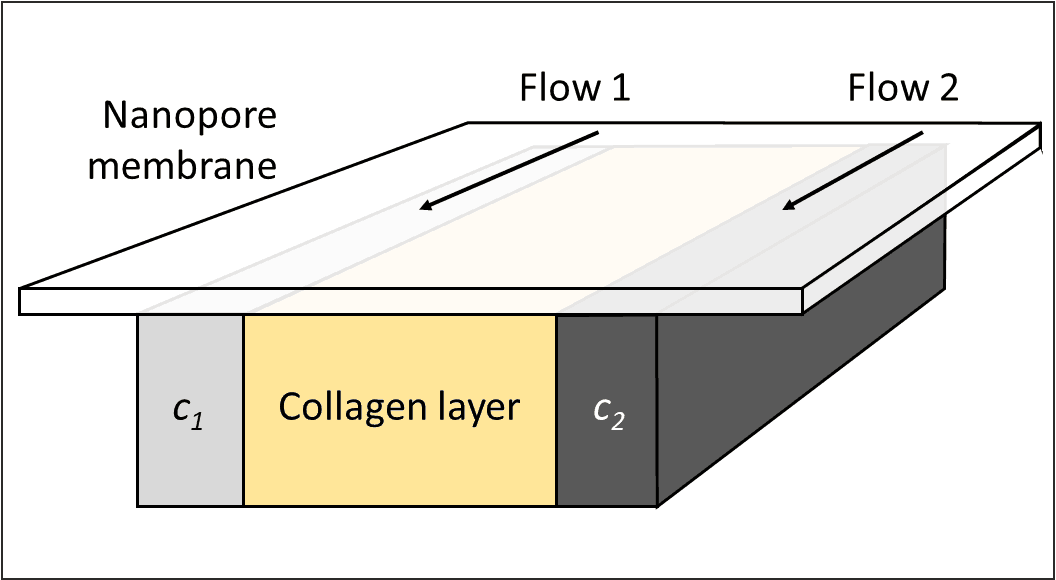
Gradient chamber principle. Two different concentrations of a substance (c1, c2) flow in two channels above this setup and diffuse through the membrane into the space underneath. Only liquids/gels and the membrane are shown.
Gradient chambers for 22×22 mm2 MicCell, with collagen channels of different width, top (left) and bottom view (middle). Through holes in the top foil, the bottom channel is filled with cell-laden collagen and the two small top channels are filled with liquids to study. Five layers are needed: top cover foil, top channel foil, pore membrane, bottom channel foil, bottom cover foil. The right picture shows the PC body with six threaded holes with O-rings, and the MicCell support.
![]()
Courtesy Tilo Pompe Lab, University of Leipzig.
Detection of Biological Polutants in Waste Water
A foil-based microfluidic flow cell based on the MicCell System has been developed for the detection of pollutants in wastewater. This project was conducted by Kurt-Schwabe-Institute Meinsberg and GeSiM. Together with other partners (BMBF project BIOSAM HIGS), we designed a biosensor setup containing this foil-based flow cell for the detection of the pharmaceutical compound diclofenac. The flow cell encloses immobilized genetically modified yeast cells that produce a fluorescent protein upon stimulation with diclofenac. Due to its special design, the flow cell allows supply with nutrient solution and analyte while preventing loss of reporter cells. Fluorescence intensity, which correlates with the diclofenac concentration, is then detected by fluorescence microscopy or a spectrometric detection unit. A change of the specification of the engineered yeast cells allows the detection of a wide panel of substances that can be parallelized for high convenience and efficiency.
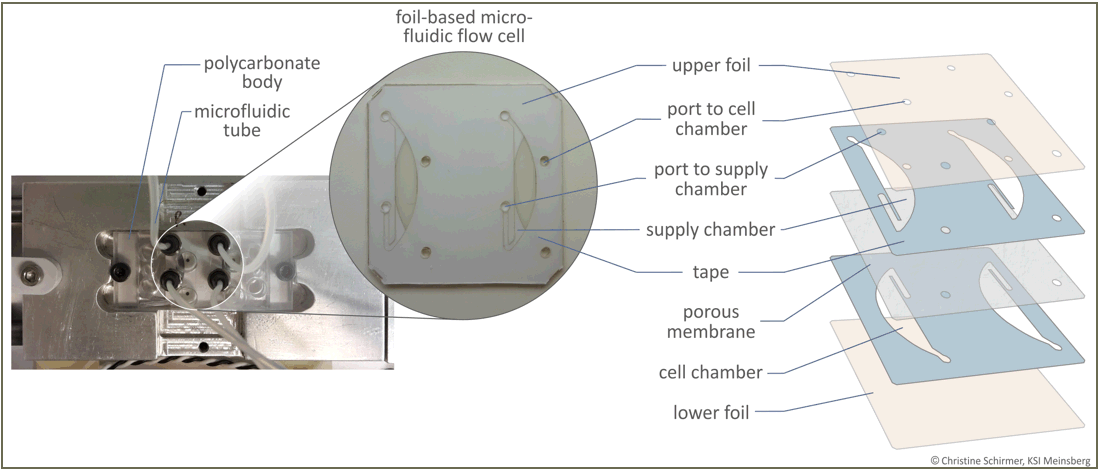
Left: Flow cell connected to the tubes via a polycarbonate body that is mounted on the detection unit. The sensor chambers are located underneath the polycarbonate body with the microfluidic tubes. Middle: Photo of the foil-based microfluidic flow cell. The dimensions of the flow cell are 22 x 22 mm, the height of the chambers is 100 µm. Right: Exploded drawing of the foil-based microfluidic flow cell.
The yeast cells are immobilized in the two lower cell chambers and covered by a porous membrane, which allows diffusion of nutrients and analytes from the supply chambers to the yeast cells but prevents them from leaving the cell chambers.
Courtesy of Kurt-Schwabe Institute Meinsberg
Schirmer et al., Talanta 203 (2019) 242–247):Portable and low-cost biosensor towards on-site detection of diclofenac in wastewater
Primary Cell Adhesion and Biofilm Formation on Anti-(bio)fouling Surfaces
In order to exploit the potential of biofilms or minimize the risk of their formation it is important to study and characterize them. To meet these aims the biomonitoring group of the Institute of Food Technology and Bioprocess Engineering at TU Dresden established a modular flow cell system with a parallel plate flow chamber. It is based on GeSiM’s MicCell and offers possibilities to study biofilms on opaque surfaces in continuous flows under a fluorescence microscope.
The centrepiece of the flow chamber is a microfluidic chip, consisting of a glass slide, a fluidic layer and the substrate. The fluidic layer is made from a polymer 3D printed on the glass slide and both its thickness and shape can be modified.
 An innovative sample holder was designed. It is made of stainless steel with holes to ensure liquid flow across the sample surface. The advantage of this setup is that samples with various geometries can be examined with no need for drilling before analyses. Hence the flow cell can be easily and rapidly adapted to meet different requirements.
An innovative sample holder was designed. It is made of stainless steel with holes to ensure liquid flow across the sample surface. The advantage of this setup is that samples with various geometries can be examined with no need for drilling before analyses. Hence the flow cell can be easily and rapidly adapted to meet different requirements.
Furthermore, due to the laminar flow inside the microfluidic channel, conditions can be tightly controlled, and we can regulate variables such as oxygen and nutrient levels simply by varying the medium supply.
The group used the flow chamber to study the adhesion behaviour of microorganisms and the first stages of biofilm formation on various materials. The acquired data were used to characterize the local and time-related biofilm formation and to derive parameters like growth rate, biofilm height and biomass volume.

Technische Universität Dresden
Faculty of Mechanical Science and Engineering
Institute for Food Technology and Bioprocess Engineering
Chair of Bioprocess Engineering, Prof. Th. Bley
Biomonitoring Group, Assoc. Prof. E. Boschke, Dipl.-Ing. S. Mulansky