Long-Lasting Ocular Implants for Posterior Eye Diseases:
Visual impairment and blindness are becoming a global issue due to their significant effects on social interactions and economic development. Diabetic retinopathy (DR) and age-related macular degeneration (AMD) are retinal disorders that are the main factors leading to blindness and vision impairment. As the elderly and diabetic populations grow, the prevalence of these conditions is expected to increase, posing challenges in reversing their progression. A timely and accurate diagnosis is essential for administering appropriate treatments to maintain or improve vision.
Current treatments, such as intravitreal injections of anti-VEGF agents, are costly and may hinder equitable healthcare access. Consequently, there is a pressing need for alternative therapies. Researchers from Queen’s University Belfast, Airlangga University and University of Seville have addressed this need by developing axitinib-loaded polymeric ocular implants to treat posterior ocular diseases, including DR and AMD.
Axitinib (AX), a potent VEGF receptor inhibitor, was incorporated into a polycaprolactone (PCL) and Precirol-based matrix. The resulting mixture was then placed into the piston extruder of the BioScaffolder 3.2 and using a semi-solid extrusion (SSE) technique, rod-shaped implants were printed. The printed implants were then cut into 6 mm lengths. The implants were characterized using FTIR spectroscopy, SEM imaging, and thermal analysis, and they were also evaluated for their drug release and biocompatibility.
The AX-loaded implants demonstrated thermal stability and no chemical interactions with the matrix components. Biocompatibility tests using ARPE-19 cells confirmed that the implants were non-toxic and safe for ocular use.
The study successfully developed axitinib-loaded polymeric ocular implants that can sustain drug release for up to 180 days and minimize side effects. These implants offer a promising alternative to frequent intravitreal injections for treating posterior ocular diseases and ocular neovascularisation.
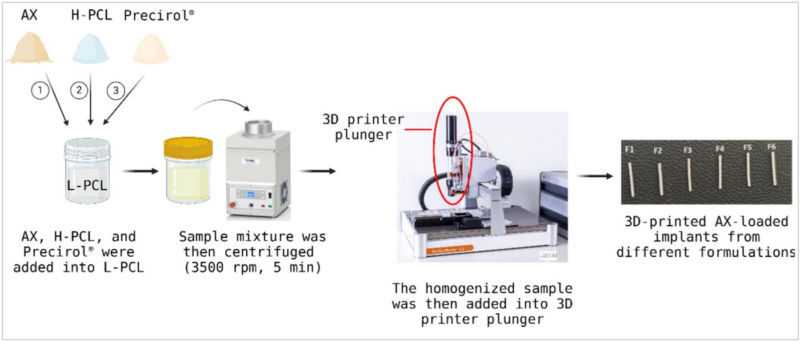
Taken from Fig. 2. Schematic diagram of the fabrication of AX-loaded implants. The implants were printed using a 0.4 mm nozzle diameter, with the applied pressure set at 300 kPa and the printing temperature at 70 °C.
This article is based on the following publication:
Annuryanti, F., Adhami, M., Abdi, U., Robles, J. D., Larrañeta, E., Vora, L. K., & Thakur R. R. S (2025). Development of axitinib-loaded polymeric ocular implants for the treatment of posterior ocular diseases. International Journal of Pharmaceutics, 669, 125099. https://doi.org/10.1016/j.ijpharm.2024.125099
